


|
 |
|
|
#1 |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Wierd Nitrate Test Results.
In an attempt to increase the sensitivity of nitrate detection with the Salifert NO3 test, I evaporated 5 mL of tank water (no nitrates detected) under a stream of air overnight. The white residue was dissolved in 1 mL distilled water and tested. The results where about 10 ppm, the expected no more than 1 ppm (assuming the original tank water sample had less than or equal to 0.2 ppm NO3 times 5). I repeated the procedure but dissolved the residue in 5 mL distilled water to reconstitute the original tank water concentrations. The test result was 2.5 ppm or a bit less than 1/5 the 10 ppm found in the first test. How does evaporating tank water give higher nitrate readings?
Search of the literature did not help. Any and all thoughts welcome to help design investigation. |
|
|

|
|
|
#2 |
|
Registered Member
Join Date: Apr 2009
Location: Ottawa, Canada
Posts: 1,642
|
Maybe the N doesn't evaporate or perhaps it evaporates at a slower rate than the water; hence the concentration would rise.
__________________
120G Mixed Reef, 28G AIO zoa/pipefish tank |
|
|

|
|
|
#3 | |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Quote:
|
|
|
|

|
|
|
#4 |
|
RC Mod
 Join Date: Mar 2002
Location: Mountain View, CA, USA
Posts: 88,616
|
There might be some bacterial activity happening. That's my first guess, but that is a large increase in nitrate. I'm not sure what's happening.
__________________
Jonathan Bertoni |
|
|

|
|
|
#5 |
|
Registered Member
Join Date: Mar 2016
Posts: 48
|
I think the problem you are facing is non-linearity w respect to the color scale. meaning, 0.2ppm is super inaccurate, but 1 or 10ppm is dead on. this could easily destroy your experiment. also, you can't increase the sensitivity of a test like this, to change sensitivity, you literally need to do different chemistry. good idea though.
|
|
|

|
|
|
#6 | |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Quote:
So, cell lysis? I will this thread with a table of test data. |
|
|
|

|
|
|
#7 | |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Quote:
The idea behind increasing the sensitivity of the test is to increase the concentration of the analyte which should be OK if something else in the sample doesn't go bad. In my initial attempt, a five fold increase in concentration gave me very much more nitrate than expected. That's why the later investigation involved reconstituting the dried sample to its original volume. I will post a table of my latest test results. |
|
|
|

|
|
|
#8 |
|
Registered Member
Join Date: Mar 2016
Posts: 48
|
can you elaborate on how you determined linearity? if 0.2 is indeed the quantitation limit, I highly highly doubt that the test is linear through that range.
|
|
|

|
|
|
#9 |
|
RC Mod
 Join Date: Mar 2002
Location: Mountain View, CA, USA
Posts: 88,616
|
Cell lysis is a reasonable hypothesis.
__________________
Jonathan Bertoni |
|
|

|
|
|
#10 | |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Quote:
|
|
|
|

|
|
|
#11 |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
I am also looking at evaporated new salt water and tank water stored at pH 12.5 overnight. The latter sample is an attempt to reproduce high pH of concentrated tank water. Can't work out why pH went up.
A sample of tank water that was stored overnight looked normal except for PO4 being 0.15 ppm instead of 0.03 ppm. Your idea of bacterial activity maybe. I totally forgot to measure NH3 level. Next time. |
|
|

|
|
|
#12 |
|
-RT * ln(k)
Join Date: Sep 2010
Location: Little Rock
Posts: 9,705
|
There's an easier way to increase the range of the test. Beer's law is A = eBC. e is epsilon here, but I can't type that. It's a constant for the dye. C is your concentration, that's the leg you are messing with right now. But B is the pathlength and it seems to me that making that larger would be easier than concentrating the sample. Rework your reagents so you can do a much larger volume and run the test in a tall container.
__________________
David Current Tank: Undergoing reconstruction... |
|
|

|
|
|
#13 | |
|
Registered Member
Join Date: Mar 2016
Posts: 48
|
Quote:
|
|
|
|

|
|
|
#14 | |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Quote:
|
|
|
|

|
|
|
#15 | |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Quote:
My definition of linearity is suitable for my needs at this time. I am looking for trends, and only interested in discovering big changes. I guess my approach might be classified as "semi-quantitative", or is that an oxymoron? |
|
|
|

|
|
|
#16 | |
|
Registered Member
Join Date: Mar 2016
Posts: 48
|
Quote:
|
|
|
|

|
|
|
#17 | |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Quote:
|
|
|
|

|
|
|
#18 |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Just to finish off this thread, here are the test results. Compare "dried" vs "stored" results for each sample type in the table. "NSW (old)" refers to new salt water that is about a month old while "NSW (new)" is freshly prepared salt water. The water was analyzed after it was "dried", "stored" in a 3 dram bottle over night or in one case the pH was increased to 12.5 overnight in a 3 dram vial before it was neutralized.
Clearly, the act of drying the salt water samples under a stream of air over a 24 hour period gave curiously high nitrate, ammonia and phosphate readings compared to just storing the same sample of water in a 3 dram vial. Just storing samples in capped vials increased the PO4 reading. I had originally hoped that concentrating tank water samples would enable me to measure lower levels of nitrates. I ended up with a bit of a mess. I will be back to this topic when I sort the phosphate measurements. I have probably stumbled on what is already known: I am likely seeing meta and some organophosphates being converted to orthophosphate. More later. 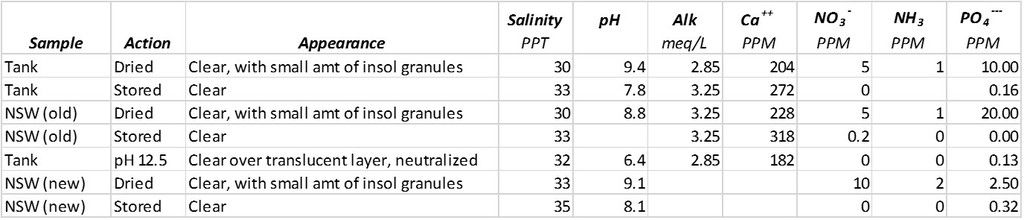
|
|
|

|
|
|
#19 |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Wierdness Possibly Solved
Over the past year, I performed enough experiments to suggest an answer. Jonathan’s suggestion looks like the winner.
When I store a capped or open 200 mL sample of tank water in the dark for a week, NH3, NO3 and PO4 levels do not change. But if air is bubbled through the sample (evaporated water replaced daily with RO/DI), NH3 and NO3 levels begin to rise between days 2 and 4. I repeated the experiment for twenty days with the same results, ending with 1 ppm NH3, 5 ppm NO3 and 0.1 ppm PO4. There didn’t seem to be a leveling off of the rate of NH3 or NO3 production. The solutions were slightly cloudy. I centrifuged the floc and examined it under a microscope. I saw very small cells. I am going to repeat the experiment one more time at 200 mL and look for a leveling off of NH3 production. Possible implications. (1) I cannot just evaporate tank water to to get a more concentrated solution of NO3 or PO4. (2) There seems to be a lot of biomass in my water. About 2 ppm of nitrogen was liberated by the end of my 20 day tank water experiment, even though nitrate and ammonia are undetectable at the start of the experiment. If nitrogen makes up ~8% of biologically derived matter, there is about 24 ppm of biomass in the water, or about 10 ppm TOC from the biomass. (3) If I get an occassional spike in nitrate level, it could just be a result of a small die off of biomass in the water rather than something in the sand (Though the origin doesn’t matter. I never “do” anything in response to NO3 spikes) |
|
|

|
|
|
#20 |
|
RC Mod
 Join Date: Mar 2002
Location: Mountain View, CA, USA
Posts: 88,616
|
That's interesting. I wonder how many other tanks have a similar amount of TOC in the water column. Maybe most of them?
__________________
Jonathan Bertoni |
|
|

|
|
|
#21 | |
|
Registered Member
Join Date: Aug 2014
Posts: 1,432
|
Quote:
Feldman in Advance Aquarist reported values for TOC in aquaria and reefs. 10 ppm is not way off from those values. |
|
|
|

|
 |
|
|